
 |
Mechanics |
 | (TOC) |
20. Hero's Fountain
 | (TOC) |
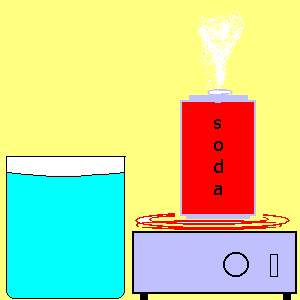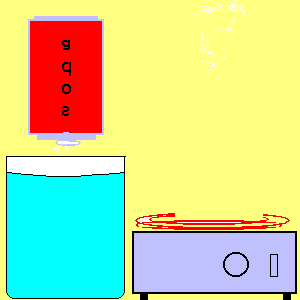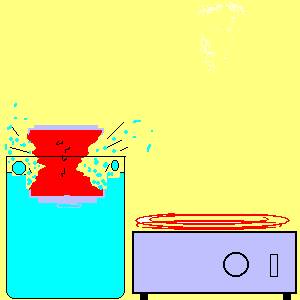
21. Atmospheric Pressure I
 | (TOC) |
22. Atmospheric Pressure II
 | (TOC) |
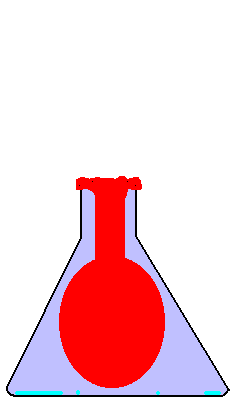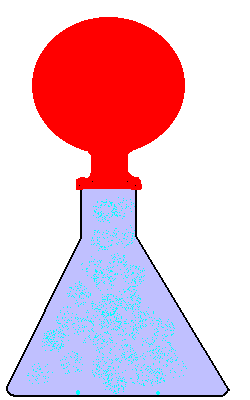
23. Flask Fun
 | (TOC) |
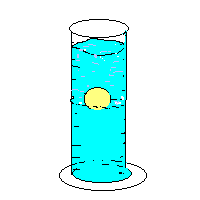
24. Cartesian Diver
 | (TOC) |
 | (TOC) |
 | (TOC) |
 | (TOC) |
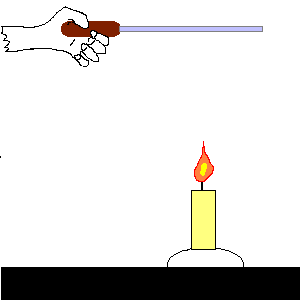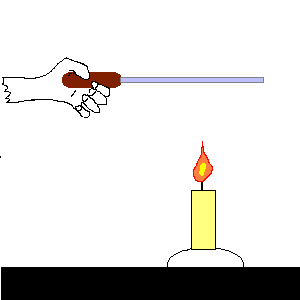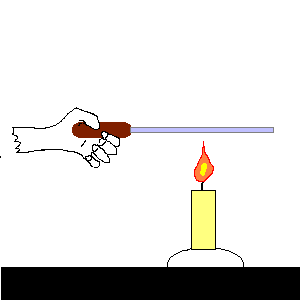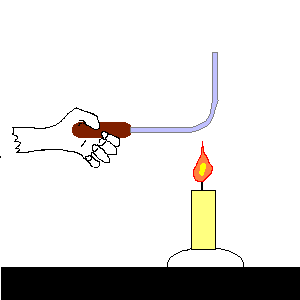 | (TOC) |
 | (TOC) |
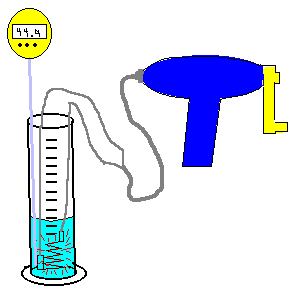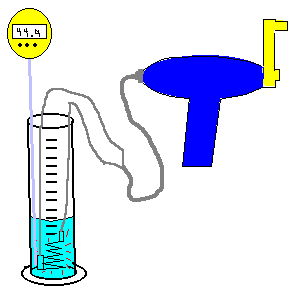 | (TOC) |
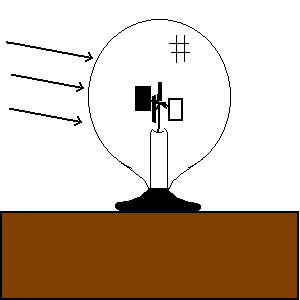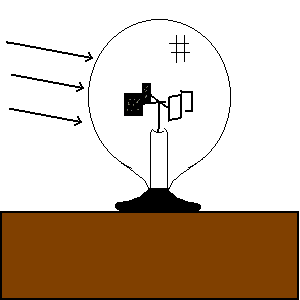 | (TOC) |
Copyright ©2000-2005 PhysicsLessons.com
If you have any suggestions, questions or comments email us!